Release time :2025-09-05
Source:support@yingchitech.com
Scan:8
The first onset of major depressive disorder (MDD) during adolescence is often associated with a lifelong risk of recurrence and functional impairment, making early and effective intervention crucial for adolescent patients with depression. However, pharmacological treatments are slow-acting and may have side effects, while psychotherapy is time-consuming and unable to rapidly alleviate symptoms. Therefore, developing safe, effective, and fast-acting treatments that significantly reduce depressive symptoms, improve patient compliance, and enhance quality of life has become a key focus of clinical research.
To address this issue, a research team from China evaluated the efficacy and safety of a five-times-daily accelerated intermittent theta burst stimulation (iTBS) protocol for non-treatment-resistant adolescents with MDD. The study was published in July 2025 in the journal Biological Psychiatry.

Study Design: Randomized, double-blind, sham-controlled clinical trial.
Participants: 74 non-treatment-resistant adolescent MDD inpatients (aged 12–18) from a child and adolescent psychiatric treatment center. Participants were randomly assigned to either an accelerated intermittent theta burst stimulation (a-iTBS) group or a sham stimulation group, with 37 participants in each group.
Stimulation Target: The left dorsolateral prefrontal cortex (DLPFC) was targeted using a TMS neuronavigation system (QuiksVision, Shenzhen Yingchi). The MNI coordinates for the stimulation site were (-44, 40, 29).
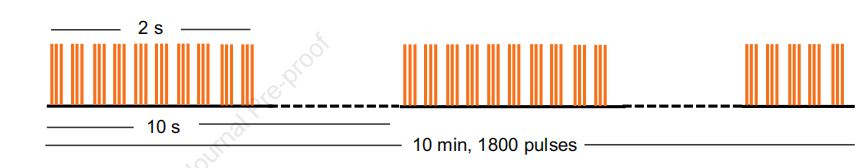
Treatment Parameters:
An electromyography (EMG) electrode was placed on the first dorsal interosseous muscle to determine the resting motor threshold (RMT). RMT was defined as the minimum stimulation intensity required to elicit a response exceeding 50 μV in at least 5 out of 10 consecutive stimuli.
a-iTBS Group:
Stimulation was delivered using a transcranial magnetic stimulation device (M-100 Ultimate, Shenzhen Yingchi) with a figure-8 coil (BY90A). The treatment consisted of five sessions per day, with each session delivering 1800 pulses at an intensity of 90% RMT. Sessions were spaced 50 minutes apart. The total treatment duration was 10 days.
Sham Stimulation Group:
A specialized figure-8 sham coil (Shenzhen Yingchi) was used to deliver sham stimulation, which replicated the same sensory experience and sound as the active stimulation without delivering effective magnetic pulses.

Clinical Assessments:
Evaluations were conducted at baseline, one day post-intervention (day 11), and at 1-month and 3-month follow-ups.
Assessment Scales:
The following standardized scales were used:
17-item Hamilton Depression Rating Scale (HAMD-17)
Hamilton Anxiety Rating Scale (HAMA)
Children's Depression Inventory (CDI)
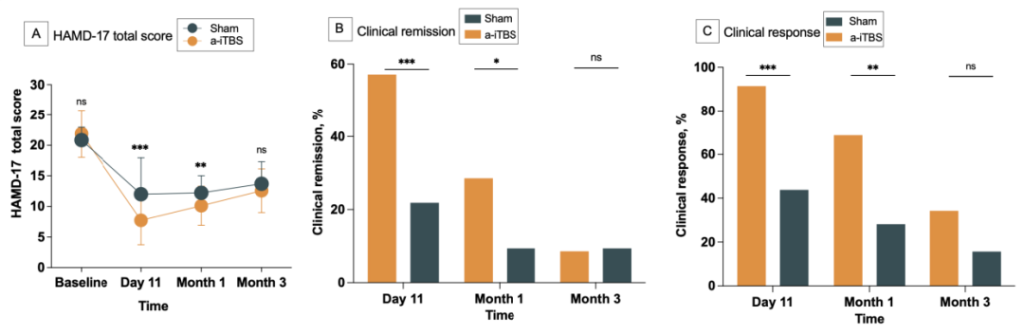
The HAMD-17 scores demonstrated statistically significant group effects, time effects, and group-by-time interactions.
One day post-intervention (day 11), the a-iTBS group showed a significantly greater reduction in mean HAMD-17 scores compared to the sham group (active: 8.09, sham: 12.28; mean difference: 4.92 points), which was statistically significant. The a-iTBS group also exhibited significantly higher remission rates (54.05% vs. 21.88%) and response rates (86.49% vs. 40.54%) than the sham group, with statistical significance.
At the 1-month follow-up, the a-iTBS group continued to demonstrate significantly lower mean HAMD-17 scores compared to the sham group (active: 10.33, sham: 12.47; mean difference: 2.14 points), which remained statistically significant. The remission (27.03% vs. 8.11%) and response rates (64.87% vs. 27.03%) were also significantly higher in the a-iTBS group than in the sham group.
By the 3-month follow-up, the mean HAMD-17 score was 12.65 in the a-iTBS group and 13.77 in the sham group, with a mean difference of 1.12 points, which was not statistically significant. Neither remission rates (8.11% vs. 8.11%) nor response rates (32.43% vs. 16.22%) showed statistically significant differences between the groups.
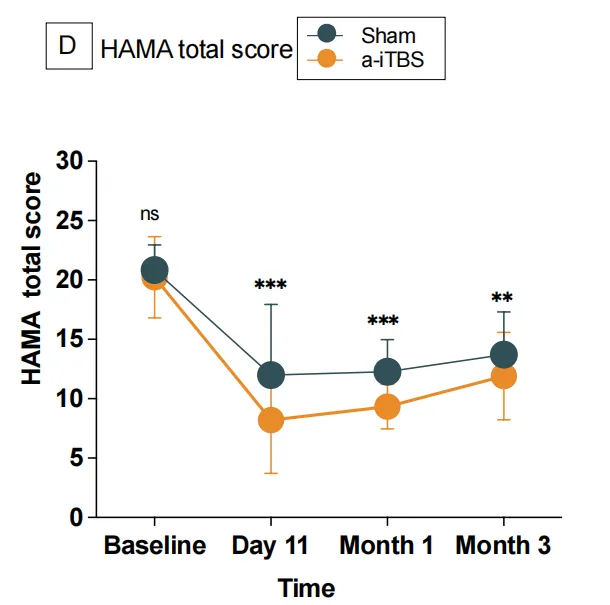
Statistically significant group effects, time effects, and group-by-time interactions were observed in HAMA scores.
One day post-intervention (Day 11):
The a-iTBS group showed a significantly greater reduction in mean HAMA scores compared to the sham group (active: 8.52, sham: 13.61; mean difference: 5.09 points, *p* < 0.001).
At the 1-month follow-up:
The a-iTBS group maintained significantly lower mean HAMA scores than the sham group (active: 9.60, sham: 12.83; mean difference: 3.23 points, *p* = 0.002).
At the 3-month follow-up:
The a-iTBS group continued to demonstrate significantly reduced mean HAMA scores compared to the sham group (active: 12.03, sham: 14.67; mean difference: 2.63 points, *p* = 0.018).
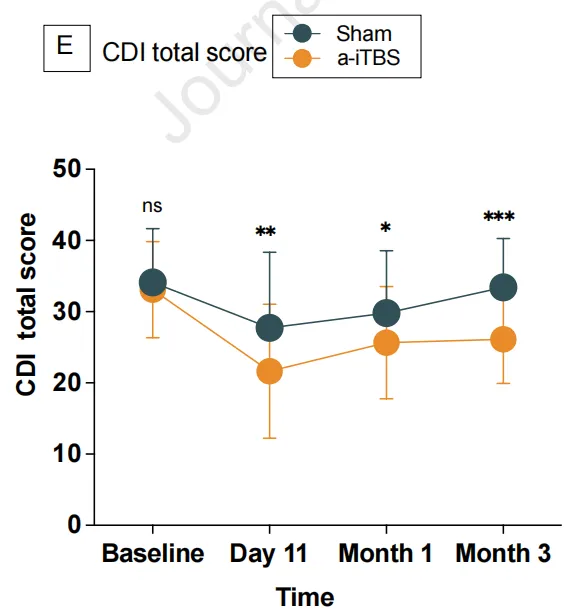
Statistically significant group effects, time effects, and group-by-time interactions were observed in CDI scores.
One day post-intervention (Day 11):
The a-iTBS group demonstrated a significantly greater reduction in mean CDI scores compared to the sham group (active: 21.28, sham: 25.67; mean difference: 4.41 points, *p* < 0.05).
At the 1-month follow-up:
The a-iTBS group maintained significantly lower mean CDI scores than the sham group (active: 25.55, sham: 29.90; mean difference: 4.36 points, *p* < 0.05).
At the 3-month follow-up:
The a-iTBS group continued to show significantly reduced mean CDI scores relative to the sham group (active: 25.38, sham: 32.93; mean difference: 7.55 points, *p* < 0.01).
The five-times-daily a-iTBS protocol administered over 10 days was found to be effective and safe for adolescents with non-treatment-resistant MDD. Future studies should focus on elucidating the underlying mechanisms of this treatment and exploring strategies to sustain its therapeutic benefits.
This content is organized by the Clinical Support Department of Shenzhen Yingchi Technology Co.,Ltd. Criticisms and corrections are welcome. For reprint, please indicate the source.
Liu X., Peng Z., Cheng F., Li G., Wang B., Hu C., Zhu Z., Hu S., Luo X., Sun J., Wang S., Fu J., Zhang W. & Zhou D., Efficacy and Safety of Accelerated Intermittent Theta-burst Stimulation for Adolescents with Major Depressive Disorder: A Randomized, Double-Blind, Sham-controlled study, Biological Psychiatry (2025).