Clinical Support Department of Shenzhen Yingchi Technology Co.,Ltd.
Peripartum Depression (PPD) is defined as the onset of depressive symptoms during pregnancy or within 12 months postpartum. PPD includes different types of depression depending on degree of severity and time of onset: prenatal depression, “baby blues” (feelings of sadness and tearfulness occurring after childbirth and usually resolving within 2 weeks of delivery) and postpartum depression.
Most of the currently available interventions for different types of postpartum depression were developed from the effective treatment of major depressive disorder (MDD), namely antidepressant drug therapy and psychotherapy, which are generally considered as first-line and second-line options for postpartum depression.
Psychotherapy alone, particularly Cognitive Behavior Therapy (CBT), has shown efficacy in treating mild to moderate depression, but may not be effective in more severe cases. In this case, a combination of psychotherapy and drug therapy is recommended. However, most antidepressants are not recommended for perinatal use, and compliance is often low due to subjective concerns about possible side effects on newborns.
In this context, novel and safe non-drug treatment options are gaining more and more attention. Repetitive Transcranial Magnetic Stimulation (rTMS), as a neuromodulation technique, uses magnetic fields to stimulate areas of the cerebral cortex, affect synaptic transmission, and change neuronal activity and connectivity. It has shown significant efficacy in the treatment of perinatal depression. In addition to curative effect, more and more people began to pay attention to the safety of TMS treatment.
A systematic review published in Psychiatry Research (IF=11.3) in May 2023 mainly discussed the safety of transcranial magnetic stimulation in the treatment of perinatal depression.

A total of 23 studies were included in this review, including 2 randomized controlled trials (RCTs) and 21 non-RCTs. Among them, 5 were open-label studies, 1 was a non-randomized case-control study, and 15 were case report studies.
In the systematically reviewed studies, high-frequency transcranial magnetic stimulation was most commonly used (11/23 studies), with a pulse number of 1000 or less being the most common daily dose (9/23 studies); the most commonly targeted brain region was the left DLPFC (11/23 studies); and the most commonly used measure of neonatal health was the APGAR score (10/23 studies).

In the review, 11 of 23 studies reported mild side effects in mothers, mainly headache, mild pain at the site of irritation and scalp discomfort.
In the iTBS study, the main side effects experienced by the mothers were the same as those known for TMS: scalp pain (which decreased in all patients during treatment), fatigue after the first stimulation, headache, dizziness, jaw twitching. Subjective memory impairment improved in all patients. iTBS during pregnancy had similar effects as iTBS in nonpregnant women, producing remission rates comparable to rTMS regimens. Therefore, as no serious side effects were reported in either mothers or children during the study period, iTBS may appear to be a more rapid treatment option, with a stimulation time of just 3 minutes.
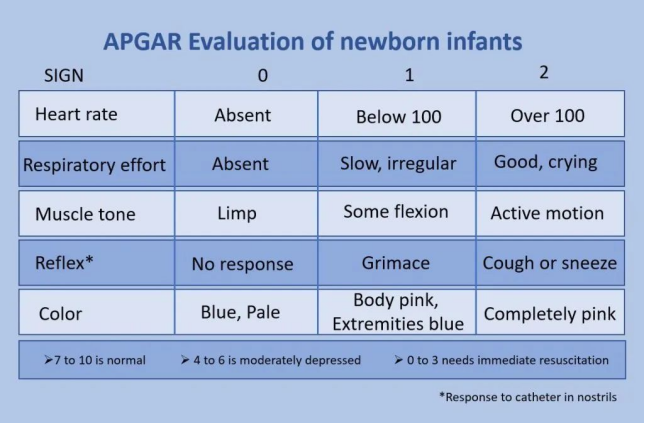
A total of 122 neonates were systematically reviewed in the review, and none of the included studies reported major adverse effects on neonates. In studies using the APGAR score, all neonates recorded an APGAR score >7.9 at 1, 5, and 10 minutes. In two studies using iTBS, one infant was large for gestational age and the other infant was born with meconium-colored amniotic fluid but had no other complications; all infants in both studies had an APGAR score of 10 after birth.
The APGAR score is a standard scoring system used to assess the health and fitness of newborns. Assessments are usually made at 1 and 5 minutes after birth. The APGAR score involves the assessment of the following five areas: heart rate, respiration, muscle tone, reflex stimulation, and appearance color. Score 7-10 points: It means that the newborn is in good condition and adapts well.
In addition, the results of 3 of these studies showed that TMS treatment after delivery had no effect on lactation and mother-infant bonding. Findings highlight the minimal dropout rate, further supporting the safety and feasibility of rTMS for PPD patients: only two studies had 2 and 1 participant dropout due to time reasons, respectively.


The importance of this literature lies in the comprehensive assessment of the use of TMS in perinatal depression through a systematic review approach, with a special focus on neonatal safety. For clinicians and researchers, this is an important reference that can help them more fully understand the risks and benefits of TMS treatment and provide direction for future research.
However, it should be noted that this literature also has some limitations. First, the sample size of the study is relatively small, and more large-scale studies are needed to further verify the conclusions. Second, there are differences in the methods and evaluation criteria used between different studies, which may affect the comparison and summary of the results.
In conclusion, this systematic review of studies helps us better understand the efficacy of TMS in the treatment of perinatal depression and explores safety issues for mothers and newborns. Although further research is needed to clarify the safety of TMS therapy, this literature provides important references for physicians and researchers to make informed decisions in practice. We look forward to more studies in the future to fill the knowledge gap in this field and provide more comprehensive and safe options for the treatment of perinatal depression.
1.This content is organized by the Clinical Support Department of Shenzhen Yingchi Technology Co.,Ltd. Criticisms and corrections are welcome. For reprint, please indicate the source.
2.Reference:
[1] Miuli A, Pettorruso M, Stefanelli G, Giovannetti G, Cavallotto C, Susini O, Pasino A, Bubbico G, De Risio L, Petta GD, Sensi SL, D'Antonio F, Martinotti G. Beyond the efficacy of transcranial magnetic stimulation in peripartum depression: A systematic review exploring perinatal safety for newborns. Psychiatry Res. 2023 May 22;326:115251. doi: 10.1016/j.psychres.2023.115251. Epub ahead of print. PMID: 37270864.
[2] 陈静,邹涛,赵丹青等.围产期精神障碍筛查与诊治专家共识[J].中国全科医学,2023,26(28):3463-3470.