Release time :2025-05-12
Source:support@yingchitech.com
Scan:452
We are thrilled to announce that YINGCHI TMS has received new FDA granted clearances for two major treatment indications:
✔ YINGCHI rTMS for Obsessive-Compulsive Disorder (OCD) – K243459
✔ Intermittent Theta Burst Stimulation (iTBS) for Major Depressive Disorder – K243460
So far, YINGCHI TMS has been approved by FDA for rTMS treating OCD, rTMS treating MDD, and iTBS for MDD.
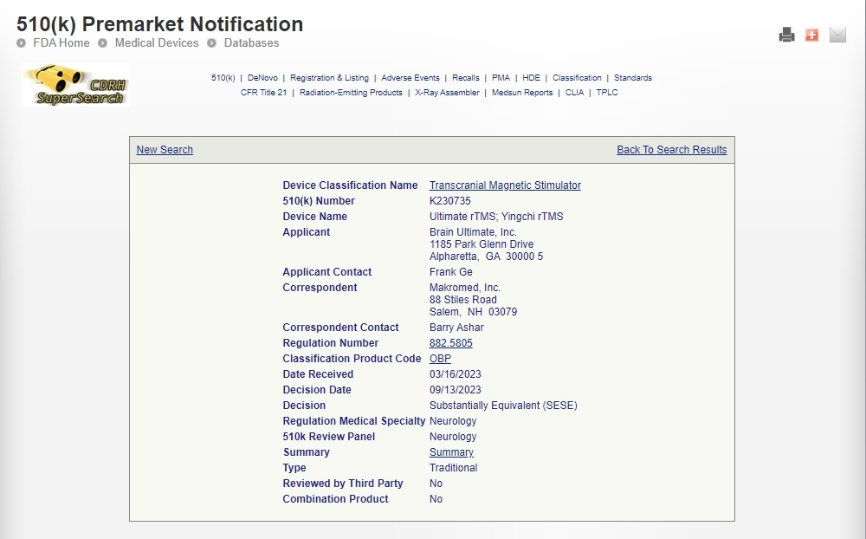
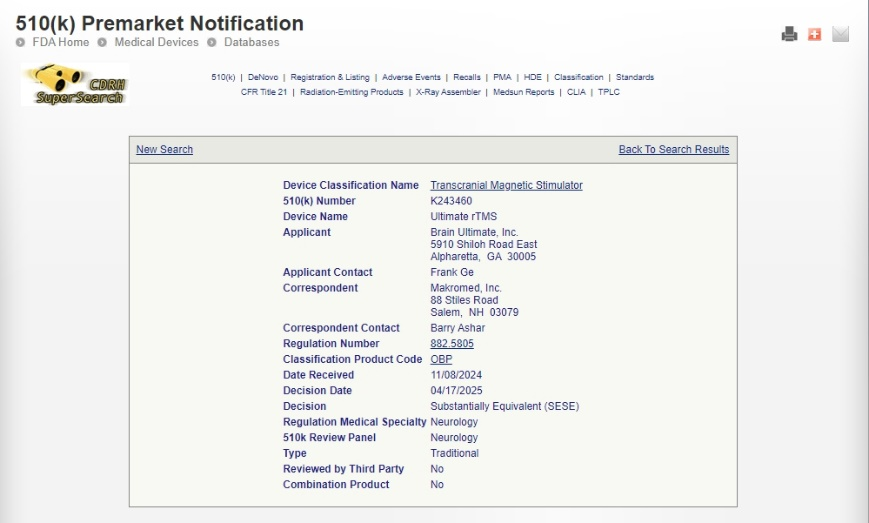
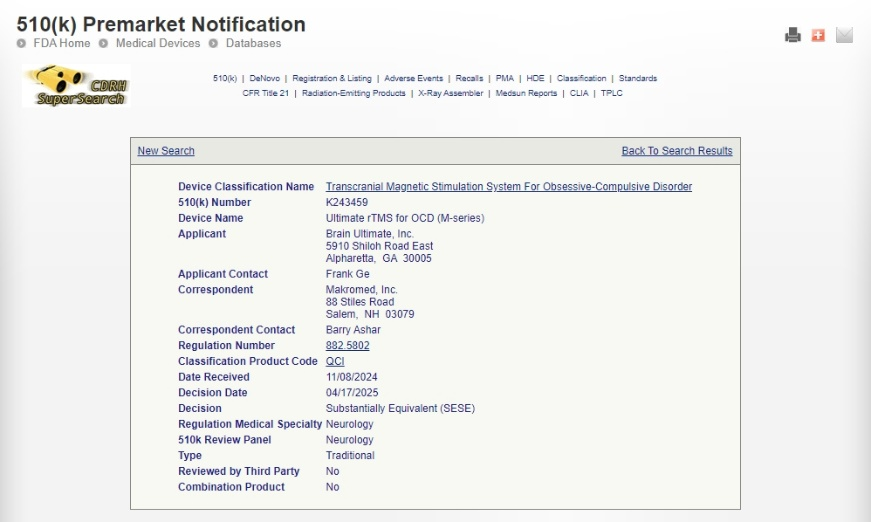
FDA clearance validates the safety and efficacy of our TMS system, making us a trusted provider of high-quality medical devices. This milestone reflects years of rigorous research, development, and collaboration with clinical experts to meet the highest regulatory standards.
With this progress, YINGCHI’s TMS device can further empower our users to address critical mental health challenges with innovative, non-pharmacological treatments. Patients now have access to shorter, highly effective iTBS sessions (as brief as 3 minutes) alongside traditional rTMS protocols, offering greater flexibility and convenience.
These FDA clearances solidify the trust that healthcare providers, patients, and partners place in our technology to deliver consistent, reliable results. We will continue to innovate and collaborate with healthcare professionals to deliver cutting-edge solutions that transform lives.

Statement
This content is organized by the Clinical Support Department of Shenzhen Yingchi Technology Co.,Ltd. Criticisms and corrections are welcome. For reprint, please indicate the source.